>> Research
- overview
- myosin
Myosin Motors
Myosin proteins are molecular motor that use the (chemical) energy stored in ATP and convert it into a mechanical displacement (kinetic energy). Since myosins can bind to actin filaments, the resulting movement is directed along those actin filaments. Most myosins move towards the plus-end of actin filaments, but there are a few exceptions.
The best-known myosins are those found in muscles, which of course allow us to walk or pick up a book. However, there are a large number of other myosins that have specific functions.
All myosins follow the same structural pattern in that they consist of three major domains: head, neck, and tail.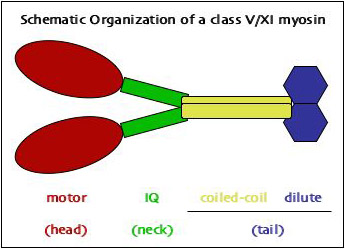
The head contains the motor domain. It binds both actin and ATP and is the catalytic core of the protein. Even though the motor domain is highly conserved, it is possible to distinguish myosins based on the head and group them into a series of sub-families (called "classes"). Currently we know of 18 classes.
The neck really is a fairly stiff alpha helix that contains a series of IQ motifs which are binding sites for calmodulin (or the related myosin light chains). The number of IQ motifs can vary between classes but is constant within a class.
The tail domain is the least conserved part of the protein. It binds to the cargo that is being moved by the myosin. In some classes, the tail also contains coiled-coil domains that allow the motor to form dimers.
More information on animal and fungal myosins can be found on the Myosin Home Page.
Plant Myosins
Plants, like all eukaryotes, also contain myosins that drive actin-based movements. In fact, actomyosin dependent cytoplasmic streaming has been described for many years and is often used as a simple microscopy exercise in introductory botany classes.
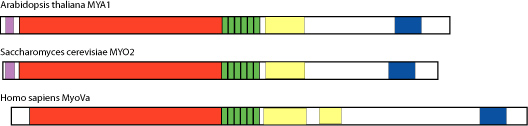
Vascular plant myosins fall only into two classes, namely VIII and XI. The model plant Arabidopsis thaliana encodes 17 myosins genes, four in class VIII, 13 in class XI (Reddy & Day 2001 Genome Biology 2:0024; see the table). Rice encodes 14 genes (2 class VIII and 12 in class XI; Jiang & Ramachandran 2004 Plant Cell Physiol 45:590). Other plants also seem to follow that pattern (Bezanilla et al. 2003 J Mol Evol 57:229).
The specific functions of these myosins is currently unknown. Based on immunolocalization data it is assumed that class VIII myosins act at the plasma membrane, possibly at plasmodesmata (Reichelt et al. 1999 Plant J 19:555; Baluska et al. 2001 Plant Physiol 126:39). Class XI myosins have been localized to organelles and are hence thought to drive organelle movement (Wang & Pesacreta 2004 Cell Motil Cytosk 57:218).
Class XI myosins have several characteristics that make them excellent organelle motors. For one, they have a domain structure that is very similar to class V myosins, which are known to drive vesicle movements in yeast and mammals.
Class XI myosins form dimers that can walk processively along actin filaments in 35 nm steps (Tominaga et al. 2003 EMBO J 22:1263). This step size is critical since it matches the helical repeat of actin filaments so that the motor can stay on one side of the filament as it pulls its organelle through the cytoplasm (see the myosin V video from the Langford group at Dartmouth). Importantly, a dimer of plant myosin XI can perform 200 steps per second, thus reaching a speed of 7 µm/s!
In vivo, we have measured speeds of individual organelles (Golgi stacks) of up to 4 µm/s (see our paper), which probably reflects the fact that the viscous cytoplasm exerts a substantial drag on the organelle. This speed is similar to that described for mitochondria and peroxisomes (many papers).
Some of the open questions we are currently addressing in our lab:
- Why do plants encode so many (putative) organelle motors?
- How much redundancy or specialization exists in the gene family?
- Do different myosins localize to different organelles?
- Are the myosin genes expressed in different tissues?
- How do the myosin proteins attach to their cargo?
- How is myosin activity and organelle motility regulated?
- Is there a larger scale coordination of organelle movements?
- Are organelle movements related to cellular polarity?
- What is the functional relevance of organelle movements?
