>> Research
Background
Research in our lab focuses on transport processes in plant cells. Transport of cellular components is essential for the delivery of newly synthesized materials to their sites of action and, more generally, for the creation of specific domains within cells that carry out distinct functions. Thus subcellular transport can be considered a prerequisite for cell polarity.
This larger research theme can be broken down into two types of transport that form interdependent delivery systems. The main focus of the lab is myosin-driven transport along actin filaments, but we also study aspects of the secretory system that delivers newly synthesized proteins to the cell surface.
Cytoplasmic Streaming, Organelle Movements, and Myosin Motors
Cytoplasmic streaming is the process of rapid movements of organelle through the cytoplasm of plant cells (see video). Although its first description dates back to the late 18th century (by Buonaventura Corti in algae), we know remarkably little about the mechanisms underlying this phenomenon or its regulation. More importantly, the physiological importance of these organelle movements are completely unknown and discussions of this subject is usually limited to the rather general concept of "mixing of cytoplasm" that supposedly is necessary in large, highly vacuolated plant cells. However, it can be expected that organelle movements (and the resulting mixing) impinge directly on the creation and maintenance of cell polarity, a fundamental process found in all cells that involves the specific placement of cellular components.
Our lab is addressing these issues by studying the molecular motors of the myosin superfamily that are thought to be driving organelle movements along actin filaments. By understanding the mechanisms underlying these movements as well as their regulation, we should be able to address the larger questions of cell polarity and streaming. In order to get a comprehensive understanding of myosin action in plants we are employing a combination of biochemical, genetic, molecular, and cell biological approaches. Among others we are pursing the following projects:
1. Myosin tail organization and organelle binding
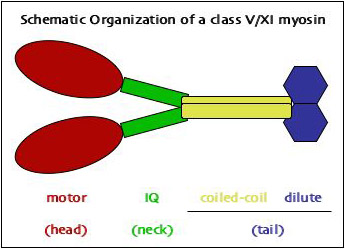 Myosin proteins consist of three domains that perform different functions (see schematic drawing at right; also see the overview over myosins on a separate page). While the first two perform the actual motor activity and act as lever arms, respectively, it is the C-terminal tail domain that provides the specificity to the actin-based movement by defining the cargo that is being moved by a specific kind of motor (see our review of motor attachment mechanisms in myosin V and XI).
Myosin proteins consist of three domains that perform different functions (see schematic drawing at right; also see the overview over myosins on a separate page). While the first two perform the actual motor activity and act as lever arms, respectively, it is the C-terminal tail domain that provides the specificity to the actin-based movement by defining the cargo that is being moved by a specific kind of motor (see our review of motor attachment mechanisms in myosin V and XI).
Of the two myosin classes present in plants both, myosin XI is similar in domain organization to myosin V in animals and fungi, suggesting similar mechanisms of cargo interaction in these related types of motors. Comparison of the globular tail domains (~400 aa) at the very C-terminus between MYA1 of Arabidopsis and Myo2p of budding yeast revealed that plant myosin XI can assume a similar conformation as myosin V, although several deletions and insertions suggest the presence of additional alpha-helices in the plant globular tail (Figure 2 - still missing). Separate in vivo localization of parts of the globular tail demonstrated that there are at least two cargo-binding sites near the C-terminus of plant myosin XI. (For details see our paper.)
While the two sub-domains as well as a reconstituted globular tail from separately expressed sub-domains can bind to organelles, we found that an intact globular tail is incapable of binding to particulate cargoes. We suspect that intramolecular dynamics prevent stable association with the organelle surface. However, organelle binding could be restored in full-tail constructs that contain both the coiled-coil domain and the globular tail. In a series of experiments involving yeast two-hybrid (Y2H) tests, co-immunoprecipitation (co-IP), bimolecular fluorescence complementation (BiFC), and Förster resonance energy transfer (FRET), we could show that dimerization (mediated by the coiled-coil region) and organelle binding (mediated by the globular tail) are interdependent processes that support each other synergistically. (For details see our paper.)
Future work includes the identification of globular tail-interacting proteins on organelle surfaces as well as determination of the amino acids involved in this interaction. Knowledge of these events will then allow us to elucidate the mechanisms that regulate organelle attachment.
2. Myosin function in root hair elongation
We have isolated several insertion mutants in myosin genes of Arabidopsis thaliana from the SALK collection that result in reduced root hair growth. Growth of this tubular extensions from epidermal cells is strictly limited to their tip and accompanied by vigorous cytoplasmic streaming. This combination of organelle movements with targeted secretion provides an excellent experimental system to investigate the role of myosins in cell polarity.
We are studying general cytoplasmic streaming by DIC optics (video will follow soon) as well as the movement of specific organelles labeled with fluorescent markers (video will follow soon). [For a detailed description of the markers used in our lab, see the organelle marker page.] Analysis of organelle movement reveals specific effects of individual myosins. We are also testing the effect of myosin mutations on a series of markers that are known to accumulate in the tip region of growing root hairs.
3. Myosin localization
We are using two basic approaches to identify the natural target of myosins. First, we are tagging various C-terminal segments of myosin coding regions with yellow fluorescent protein and express these constructs transiently in plant cells. Second, we have generated polyclonal antibodies against several peptides that reside on the surface of the globular tail domain. These peptide sequences show substantial variation among myosin paralogs and should allow us to distinguish between these isoforms.
The Secretory System of Plant Cells
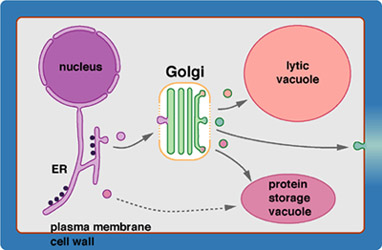 The secretory system of eukaryotic cells produces a wide range of products destined for the cell surface. It consists of a series of membrane-bounded organelles, namely the endoplasmic reticulum, the Golgi apparatus, intermediate sorting compartments, and a whole range of transport vesicles that shuttle the products (and membranes) between the larger compartments. The Golgi apparatus plays a central role in this secretory pathway. Besides its well-known role in modification of N-linked oligosaccharides, which were generated in the ER, it is also involved in the synthesis and modification of membrane lipids and, in plants, the de novo synthesis of cell wall matrix polysaccharides, the pectins and hemicelluloses. In addition, the Golgi is also a complex sorting station that continuously recycles resident ER and Golgi proteins back to their correct membranes while product molecules are allowed free passage to the target organelles.
The secretory system of eukaryotic cells produces a wide range of products destined for the cell surface. It consists of a series of membrane-bounded organelles, namely the endoplasmic reticulum, the Golgi apparatus, intermediate sorting compartments, and a whole range of transport vesicles that shuttle the products (and membranes) between the larger compartments. The Golgi apparatus plays a central role in this secretory pathway. Besides its well-known role in modification of N-linked oligosaccharides, which were generated in the ER, it is also involved in the synthesis and modification of membrane lipids and, in plants, the de novo synthesis of cell wall matrix polysaccharides, the pectins and hemicelluloses. In addition, the Golgi is also a complex sorting station that continuously recycles resident ER and Golgi proteins back to their correct membranes while product molecules are allowed free passage to the target organelles.
1. Golgi stack integrity
A central organelle of the secretory pathway is the Golgi apparatus that functions as a biosynthetic as well as a sorting compartment (for more background, see our review). It consists of a series of flattened membrane compartments, the cisternae, that contain different enzymes. We are using live cell imaging approaches to test the integrity of the stack during movements and drug treatments (e.g., compare our paper on BFA effects on Golgi stacks).
