>> Research
Background
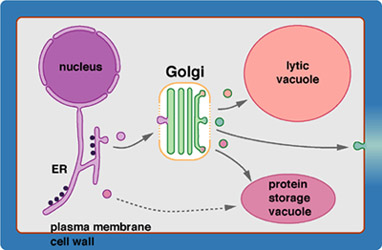 The secretory system of eukaryotic cells produces a wide range of products destined for the cell surface. It consists of a series of membrane-bounded organelles, namely the endoplasmic reticulum, the Golgi apparatus, intermediate sorting compartments, and a whole range of transport vesicles that shuttle the products (and membranes) between the larger compartments. The Golgi apparatus plays a central role in this secretory pathway. Besides its well-known role in modification of N-linked oligosaccharides, which were generated in the ER, it is also involved in the synthesis and modification of membrane lipids and, in plants, the de novo synthesis of cell wall matrix polysaccharides, the pectins and hemicelluloses. In addition, the Golgi is also a complex sorting station that continuously recycles resident ER and Golgi proteins back to their correct membranes while product molecules are allowed free passage to the target organelles.
The secretory system of eukaryotic cells produces a wide range of products destined for the cell surface. It consists of a series of membrane-bounded organelles, namely the endoplasmic reticulum, the Golgi apparatus, intermediate sorting compartments, and a whole range of transport vesicles that shuttle the products (and membranes) between the larger compartments. The Golgi apparatus plays a central role in this secretory pathway. Besides its well-known role in modification of N-linked oligosaccharides, which were generated in the ER, it is also involved in the synthesis and modification of membrane lipids and, in plants, the de novo synthesis of cell wall matrix polysaccharides, the pectins and hemicelluloses. In addition, the Golgi is also a complex sorting station that continuously recycles resident ER and Golgi proteins back to their correct membranes while product molecules are allowed free passage to the target organelles.
In the past, the description of the secretory system in plants was largely confined to structural studies (reviewed in Staehelin and Moore, 1995), however, recent years have seen a rapid increase in identified proteins involved in either trafficking or enzymatic modifications (Nebenführ and Staehelin, 2001). While it has become clear that the basic machinery of the secretory system in plants is similar to that of other eukaryotes, it is important to emphasize that a number of distinct differences exist which likely reflect the different structural organization and functional requirements of plant cells (Nebenführ and Staehelin, 2001). For example, the plant Golgi apparatus is organized into a large number of independent stacks that are distributed throughout the cytoplasm and can move along actin filaments (Nebenführ et al, 1999). This dynamic localization likely requires different transport mechanisms both to and from the Golgi when compared to the well-studied mammalian systems. Similarly, the important role of the Golgi in producing cell wall material (Driouich at al, 1993) puts different constraints on the flow of material through the stacks. The research in our lab is aimed at understanding some of these plant-specific adaptations of the secretory systems.
Golgi stack mobility - mechanism and implications
 To understand the dynamic transport processes surrounding the Golgi apparatus in plant cells it is necessary to employ markers that allow easy tracking of specific intracellular structures. The marker for Golgi membranes used in our lab is a fusion between a Golgi-resident mannosidase (GmMan1) and green fluorescent protein (GFP) (see schematic at right). Expresssion of this fusion protein in tobacco BY-2 suspension-cultured cells results in bright green fluorescent spots (see confocal images) that display directed movements through the cytoplasm (see movement videos). This movement is driven by the acto-myosin system, since both actin and myosin inhibitors block the movement, whereas microtubule dugs have no effect on the movement. Detailed analysis of the Golgi stack movements suggests that these are active movements with the stacks directly or indirectly coupled to motor proteins (myosins) that travel along cytoskeletal tracks (F-actin) (see panel A of figure below) (Nebenführ et al, 1999).
To understand the dynamic transport processes surrounding the Golgi apparatus in plant cells it is necessary to employ markers that allow easy tracking of specific intracellular structures. The marker for Golgi membranes used in our lab is a fusion between a Golgi-resident mannosidase (GmMan1) and green fluorescent protein (GFP) (see schematic at right). Expresssion of this fusion protein in tobacco BY-2 suspension-cultured cells results in bright green fluorescent spots (see confocal images) that display directed movements through the cytoplasm (see movement videos). This movement is driven by the acto-myosin system, since both actin and myosin inhibitors block the movement, whereas microtubule dugs have no effect on the movement. Detailed analysis of the Golgi stack movements suggests that these are active movements with the stacks directly or indirectly coupled to motor proteins (myosins) that travel along cytoskeletal tracks (F-actin) (see panel A of figure below) (Nebenführ et al, 1999).
 The movement of Golgi stacks has several implications for the functioning of this organelle. One aspect is the remarkable stability of the stacks despite their rapid movements through the viscous environment of the cytoplasm. Presumably this requires strong cohesive forces that keep the cisternae together. Another aspect is that transport vesicles that deliver secretory cargo to and from the Golgi have to contend with a moving target. We have proposed a model that attempts to combine the experimental observations with a mechanism that would explain efficient exchange of transport vesicles between ER and Golgi. In particular, we postulate that Golgi stacks have to stop at ER export sites in order to ensure uptake of vesicles ("recruitment model", Nebenführ et al., 1999). The model assumes a local stop signal that is produced in areas where transport to or from the Golgi has to occur (see panels B and C of figure on left). By preventing Golgi stacks from leaving an area, these postulated stop signals would allow for higher efficiency of diffusion limited vesicular transport.
The movement of Golgi stacks has several implications for the functioning of this organelle. One aspect is the remarkable stability of the stacks despite their rapid movements through the viscous environment of the cytoplasm. Presumably this requires strong cohesive forces that keep the cisternae together. Another aspect is that transport vesicles that deliver secretory cargo to and from the Golgi have to contend with a moving target. We have proposed a model that attempts to combine the experimental observations with a mechanism that would explain efficient exchange of transport vesicles between ER and Golgi. In particular, we postulate that Golgi stacks have to stop at ER export sites in order to ensure uptake of vesicles ("recruitment model", Nebenführ et al., 1999). The model assumes a local stop signal that is produced in areas where transport to or from the Golgi has to occur (see panels B and C of figure on left). By preventing Golgi stacks from leaving an area, these postulated stop signals would allow for higher efficiency of diffusion limited vesicular transport.
 The recruitment model predicts that Golgi stacks should accumulate in regions where active transport to and/or from the Golgi occurs. This is true for cytokinesis when essentially all Golgi products are directed to the forming cell plate that separates the daughter cells (Nebenführ et al, 2000). Comparison of GFP-labeled Golgi stacks with mitochondria and plastids (labeled with the red MitoTracker dye) demostrates that plant cells are able to segregate their organelles during mitosis (see image on right). Specifically, Golgi stacks are recruited to the spindle poles and the "Golgi belt", a narrow band of cortical cytoplasm that faithfully predicts the future site of cell division.
The recruitment model predicts that Golgi stacks should accumulate in regions where active transport to and/or from the Golgi occurs. This is true for cytokinesis when essentially all Golgi products are directed to the forming cell plate that separates the daughter cells (Nebenführ et al, 2000). Comparison of GFP-labeled Golgi stacks with mitochondria and plastids (labeled with the red MitoTracker dye) demostrates that plant cells are able to segregate their organelles during mitosis (see image on right). Specifically, Golgi stacks are recruited to the spindle poles and the "Golgi belt", a narrow band of cortical cytoplasm that faithfully predicts the future site of cell division.
Open questions
A fundamental question that is still unanswered is why Golgi stacks move at all. While the recruitment model suggests a role for Golgi stack movement in the distribution of its products (i.e. Golgi stacks as "delivery vehicles"), it is important to emphasize that to date this is just a hypothesis that has to be tested experimentally. Several approaches are available that will provide additional information that may ultimately help solve the puzzle. We are pursuing two complementary avenues that attack the problem from different angles. The first entails the identification and characterizarion of the motor protein that is responsible for Golgi movement. By elucidating the regulatory mechanisms that govern its activity, it is hoped that the cellular signals that control Golgi stack movement and accumulation can be identified. More details on this approach can be found on the Myosin Page. The other approach is to study the dynamics of Golgi stacks in relationship to other elements of the secretory pathway. To this end we will create additional in vivo markers for different organelles and observe them in living cells. Finally, the relationship between Golgi stacks and targeted secretion to the plasmamembrane will be studied in easily accessible tissues that display prominent asymmetric features at their cell surfaces (e.g. guard mother cells, endodermis, epidermal cells, etc.).
References
Driouich A, Faye L, Staehelin LA (1993) The plant Golgi apparatus: a factory for complex polysaccharides and glycoproteins. Trends Biochem Sci 18: 210-214
A. Nebenführ, L. Gallagher, T.G. Dunahay, J.A. Frohlick, A.M. Mazurkiewicz, J.B. Meehl, L.A. Staehelin (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiology 121, 1127-1141. [abstract] [pdf, 1.5 MB]
A. Nebenführ, J.A. Frohlick, L.A. Staehelin (2000) Redistribution of Golgi stacks and other organelles during mitosis and cytokinesis in plant cells. Plant Physiology, 124, 135-151. [abstract] [pdf, 1.1 MB]
A. Nebenführ, L.A. Staehelin (2001) Mobile factories: Golgi dynamics in plant cells. Trends in Plant Science, 6:160-167. [abstract] [pdf, 130 kB]
Staehelin LA, Moore I (1995) The plant Golgi apparatus: Structure, functional organization and trafficking mechanisms. Annu Rev Plant Physiol Plant Mol Biol 46: 261-288
